Prospective Feasibility Study for Using Cell-Free Circulating Tumor DNA–Guided Therapy in Refractory Metastatic Solid Cancers: An Interim Analysis
Key Findings
-
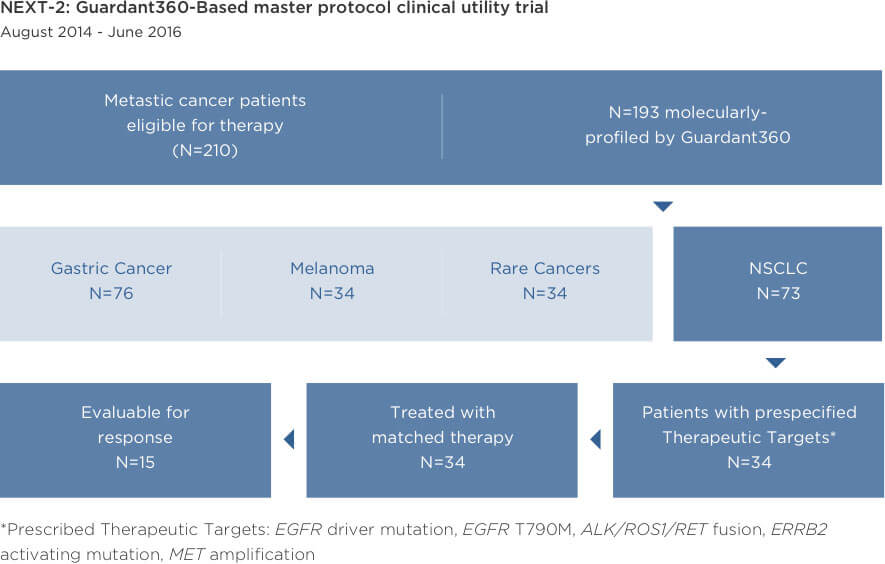
Comprehensive ctDNA testing can effectively guide therapy selection
-
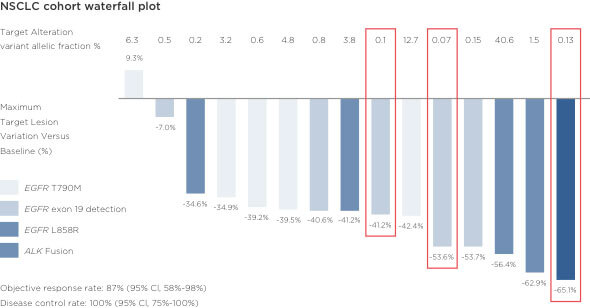
Response rates to therapy selected based on Guardant360 results were consistent with those in tissue-based targeted therapy studies
"Among patients with insufficient tumor tissue for sequencing, ctDNA testing can be a feasible option to guide molecularly matched therapy."
Seung Tae Kim, MD