Genomic Profiling of Advanced Non–Small Cell Lung Cancer in Community Settings: Gaps and Opportunities
Key Findings
-
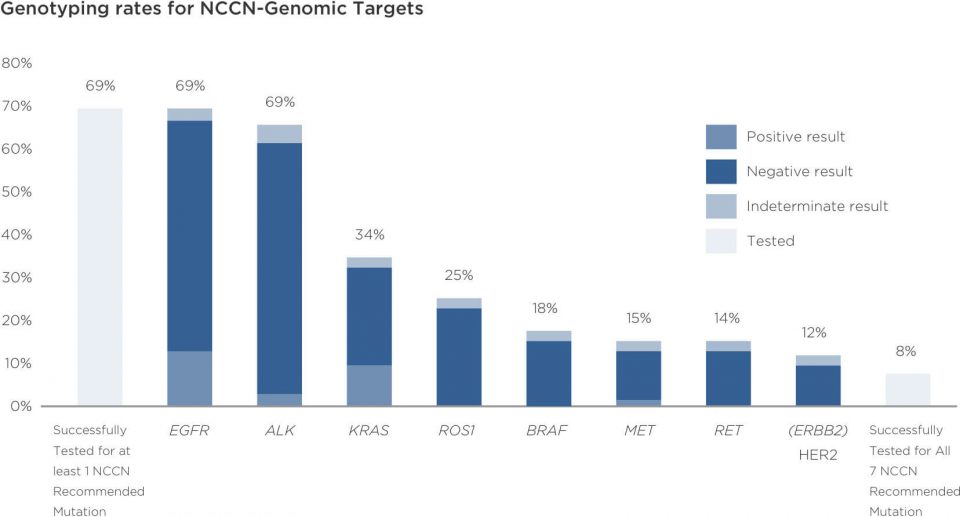
The demands on tissue specimens from diagnostic stains and PD-L1 testing may leave little remaining for genomic profiling
-
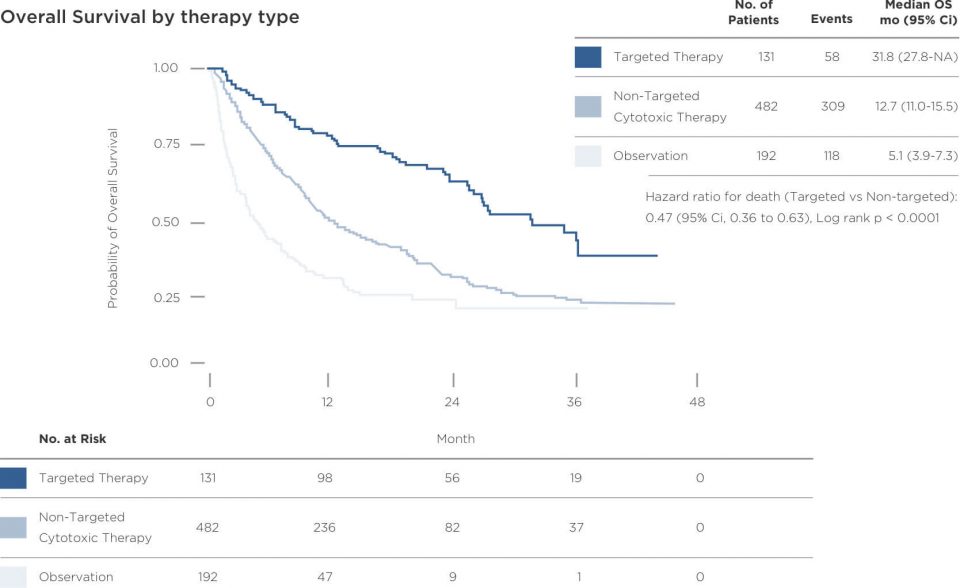
Gaps in genomic biomarker testing can be overcome with new technologies
"Liquid biopsy could help rescue patients who cannot receive genotype testing because of insufficient tissue."
Martin E. Gutierrez, MD