Key Findings
-
Concordance between Guardant360 and tissue testing was greater than 90% for the four biomarkers with FDA-approved therapies
-
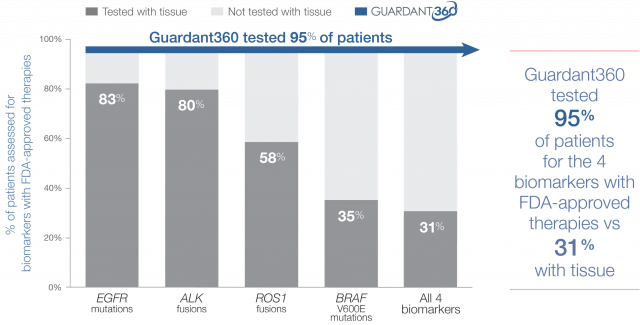
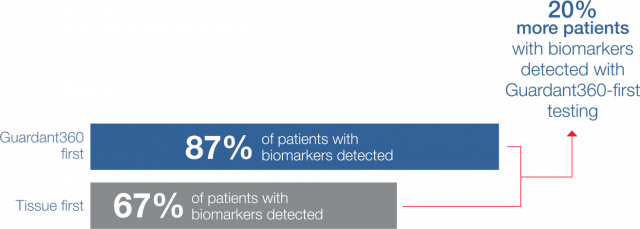
Guideline-recommended biomarker testing was completed for 95% of patients with Guardant360 vs 31% with standard-of-care tissue testing
-
Turnaround time was ~1 week faster with Guardant360 vs. SOC tissue testing